Difference Between Endpoint and Equivalence Point : If you are preparing for the NEET exam, you must be familiar with the concept of titration, a common technique used in analytical chemistry to determine the concentration of an unknown solution. Titration involves adding a known solution (titrant) to an unknown solution (analyte) until a chemical reaction is complete. But how do you know when the reaction is complete?
This is where the terms endpoint and equivalence point come in. In this article, we will explain the difference between endpoint and equivalence point, and how to identify them using indicators or pH meters. Understanding the difference between endpoint and equivalence point is essential for performing accurate titrations and solving related problems in the NEET syllabus .
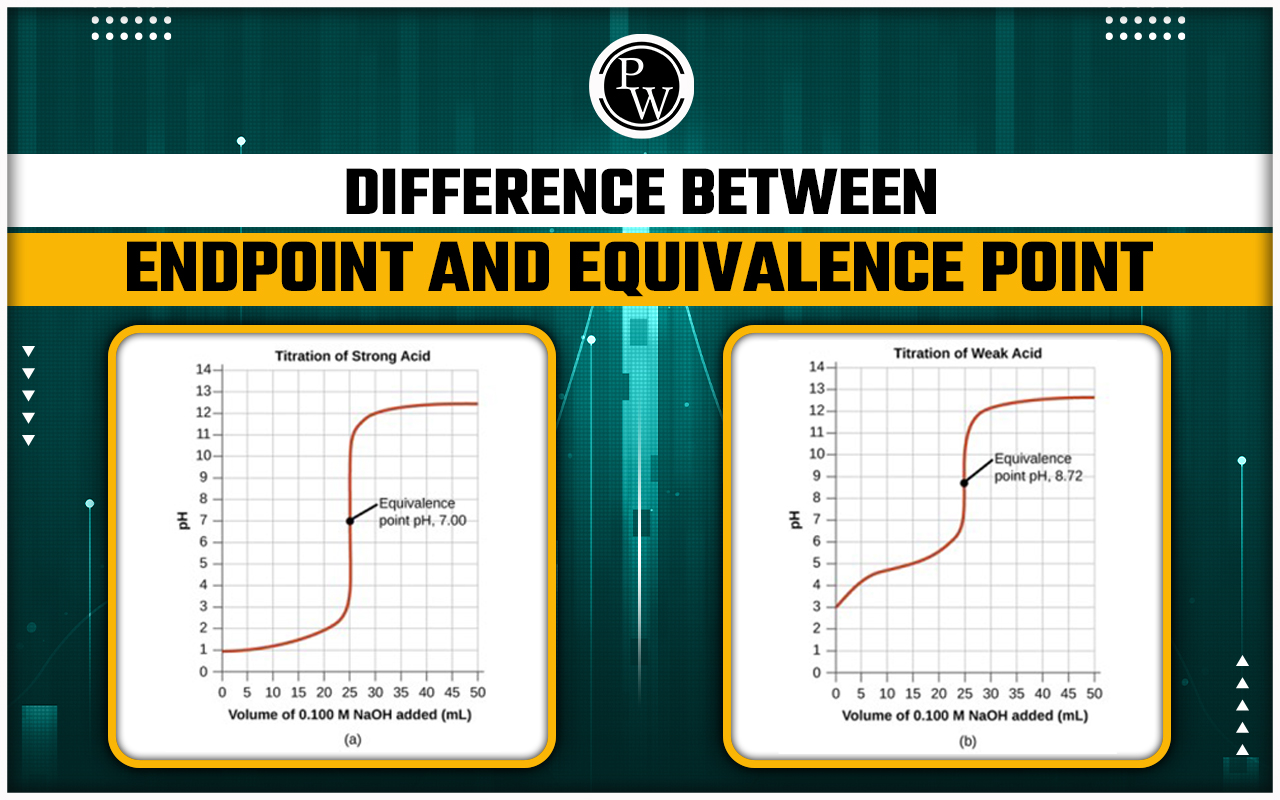
The difference between endpoint and equivalence point is one of the most important concepts in titration, a technique used to measure the concentration of an unknown solution. The equivalence point is the exact point where the amount of titrant added is equal to the amount of analyte in the sample, according to the stoichiometry of the reaction. This means that the reactants have completely converted into products, and the solution is neutral or at the desired pH. The endpoint, on the other hand, is the point where the indicator changes color, signaling the completion of the titration. The indicator is a substance that has a different color in acidic and basic solutions, and it is chosen to match the expected equivalence point. Ideally, the endpoint and the equivalence point should coincide, but in reality, there is usually a small difference between them. This difference is called the titration error, and it can affect the accuracy of the results. To minimize the titration error, one should use a suitable indicator, a sensitive pH meter, and a precise burette.
|
Difference Between Endpoint and Equivalence Point |
||
| Parameters | Endpoint | Equivalence Point |
| Definition | the titration point at which the indicator changes color to indicate that the reaction is finished | The point at which the moles of a standard solution (titrant) are stoichiometrically equivalent to the moles of the analyte being titrated |
| Indicator Use | Relies on indicators to detect the completion of the reaction | May or may not use indicators; can be determined by measuring a physical property such as pH or conductivity |
| Chemical Reaction | Does not necessarily coincide with the stoichiometric equivalence point | Coincides with the stoichiometric equivalence point, indicating the completion of the reaction |
| Visual Observation | Observable color change in the presence of an indicator | May not have a visible change; requires monitoring physical properties or using an indicator |
| Reversibility | Often associated with reversible reactions | Typically occurs in irreversible reactions |
| Determination in titration | Marks the practical endpoint of titration | Represents the theoretical completion of the reaction based on stoichiometry |
| Precision in analysis | May have uncertainties due to the subjective judgment of color change | Generally more precise, especially in quantitative analysis, as it is based on the stoichiometry of the reaction |

